Tools to foster industrial applicability and acceptance of in vitro.
SiMPLInext SA today announces its P-line products, for immediate release, comprising a complete set of complementary products from its SiMPLI & CoMPLI product families for industrial autonomous automatic TEER (Transepithelial Electrical Resistance) measurements of in vitro reconstituted human biological barriers, and pre-announces a range of its Q-line products, for release in 2019 Q1. The wellplate slide-in acquisition module is the key component of the first preclinical IoT platform for in vitro qualified industrial human tissue. SiMPLInext Press Release 04 December 2018.pdf
Here is the list of the announced product lines:
SiMPLInext Product Announcement 04 December 2018.pdf
Since its inception in the mid 1950s and widespread use in the 80s, the cell culture insert, otherwise known as Transwell™, has enabled a vast range of discoveries and applications.
To biologists who grow tissue on multiwell plate inserts, SiMPLI is a drop-in replacement that significantly extends the range of in-vitro capabilities in multiple directions:
- Transport and permeability studies of large molecules, nanoparticles in particular
- Permeability studies through non cellular membranes such as mucuses or biofilms
- Transport studies of non soluble lipophilic chemicals
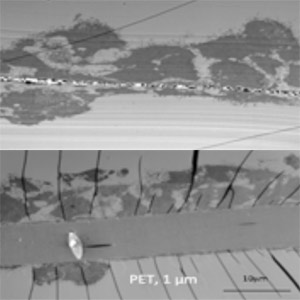
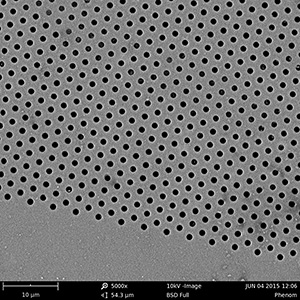
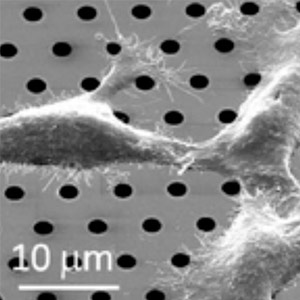
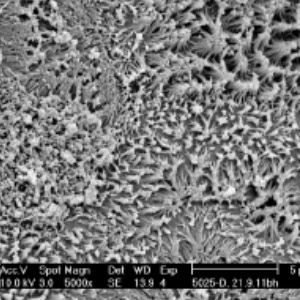
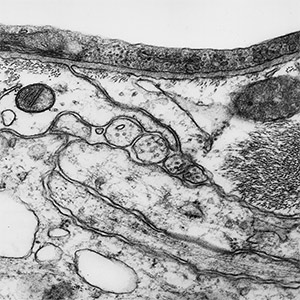






 2010: A first generation machined stainless steel prototype made it possible to clamp and seal the silicon nitride membrane using an o-ring.
2010: A first generation machined stainless steel prototype made it possible to clamp and seal the silicon nitride membrane using an o-ring.